
H NMR Correlation 1


|
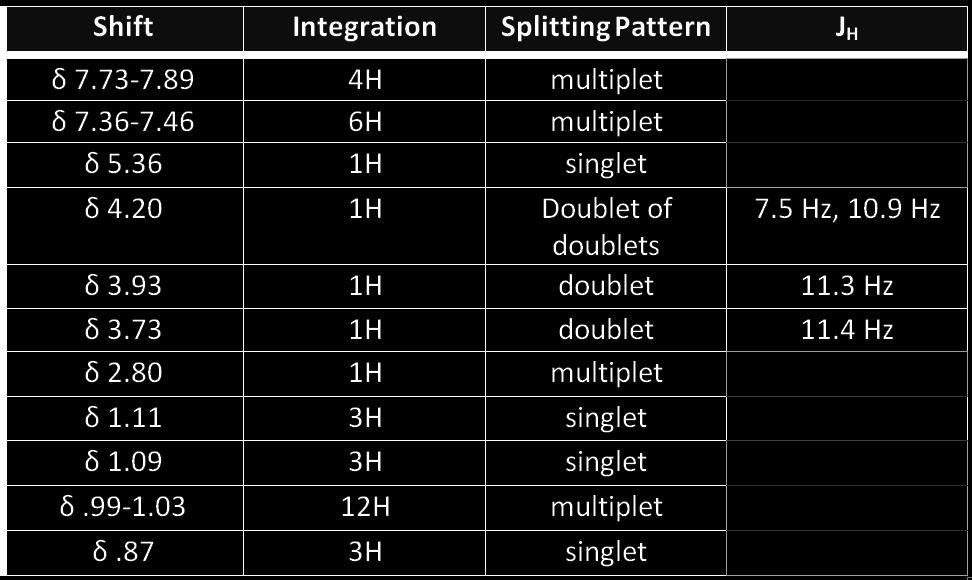
A) The first peaks, 7.73-7389 ppm, correspond to the hydrogens of a benzene ring, which is why it shows in this range. They are downfield because they are in the deshielding region of the benzene ring, which takes away more electron density. The multiplet is observed because the hydrogens couple with more than just it’s three bond neighbors.
B)The second peaks, 7.36-7.46 ppm, correspond to the rest of the hydrogens of the benzene ring. They are slightly more downfield because they are slightly farther out from the rest of the molecule. Again, multiplets are observed because they couple with more than just their three bond neighbors.
C) The third peak at 5.36 ppm corresponds to a hydrogen attached to the double bond of a pentene ring. The double bond draws electron density, which is why we observe a downfield hydrogen. It does not couple with any other hydrogens which explains the singlet peak.
D) The fourth peak at 4.20 ppm corresponds to a hydrogen attached to the carbon on which the OTBDPS group is attached. This hydrogen is downfield because of the electronegative oxygen. A doublet of doublets is observed because of the two different hydrogens close by.
E) The hydrogen at 3.93 ppm is attached to a carbon right next an electronegative oxygen atom. This will draw electron density away and that is why we observe a downfield ppm. It is a doublet because it couples with f, it’s two bond neighbor.
F) The hydrogen at 3.73 ppm is affected by similar effects as hydrogen e. It is attached to carbon right next an electronegative oxygen atom. It also shows a doublet because it couples with e.
G) The 7th peak at 2.80 ppm corresponds to the hydrogen attached to one of the cyclohexane rings, near the carbonyl. We observe a multiplet because it couples with more than just it’s 3 bond neighbors. It is slightly more downfield because it is near the double bonded oxygen near by which takes away some of the electron density.
H) The hydrogen at shift value of 1.11 ppm corresponds to hydrogens found on the methyl group attached to the carbon of the cyclopentane ring. These hydrogens have no neighbors and therefore appear as a singlet. They reflect values for a methyl group around 1.0 ppm.
I) These hydrogens are similar to group h, has no neighbors which explains the singlet peak and is in the same environment of h which is why it reflects a very close value to h, a typical methyl peak.
J) The hydrogens at .99-1.03 ppm are all located on methyl groups as part of the OTBDPS substituent and the methyl group near the OTDBPS group. These methyl groups are typical of methyl peaks around 1.0 ppm, with nothing nearby to take away it’s strong electron densities.
K) The hydrogens at .87 ppm is the methyl group inbetween two cyclohexanes rings, and reflects an even smaller ppm because it is far away from any electron density withdrawing groups. It is surrounded by similar C-H groups and has a high electron density. There are no hydrogen neighbors which is why we observe a singlet peak.